24小时服务热线:19103801095
NEWS CENTER
Recommend case
contact us

2024年1-6月审结转出注册项目审评用时情况
Review time of submissions for registration completed and transferred in January to June 2024
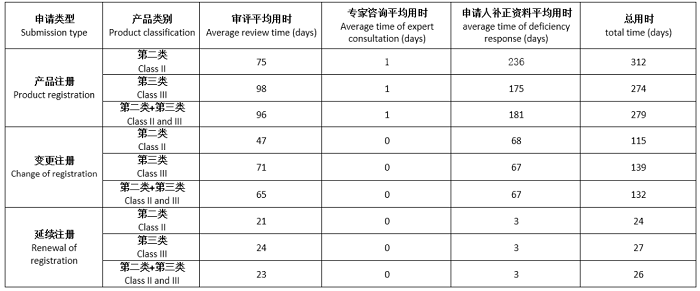
备注:
1.本表涉及时间均为工作日。
The time in this table is working days.
2.补正资料平均用时=补正资料总用时/相关项目审结转出总数量
average time of deficiency response = total time of deficiency response /total number of completed and transferred submissions with deficiencies.
3.总用时=审评平均用时+专家咨询平均用时+补正资料平均用时
Total time = average review time+ average time of expert consultation + average time of deficiency response.
4.第二和第三类产品平均用时=第二类和第三类产品用时/第二类和第三类产品总数量
Average time for Class II and III products = total treview time of class II and III products/the total number of class II and III product submissions.
5.应急审评用时不在统计范围内
Time of emergency review is out of the scope of this statistics.
站点声明:
本网站所提供的信息仅供参考之用,并不代表本网赞同其观点,也不代表本网对其真实性负责。图片版权归原作者所有,如有侵权请联系我们,我们立刻删除。如有关于作品内容、版权或其它问题请于作品发表后的30日内与本站联系,本网将迅速给您回应并做相关处理。
北京飞速度医疗科技有限公司专注于医疗器械、诊断试剂产品政策与法规规事务服务,提供产品注册申报代理、临床合同(CRO)研究、产品研发、GMP质量辅导等方面的技术外包服务。